Are you ready for Brexit? Do you have a Brexit contingency plan?
You may need either an EU/EC European authorized representative based in EU-27 countries or a UK authorised representative (so-called "UK Responsible Person") based in UK, or may even need both EU & UK representatives, depending on different brexit scenarios.
Register/Notify your MD-Medical Devices & IVD-In Vitro Diagnostic Medical
Devices with MHRA in UK & other EEA (EU/EFTA) authorities by world-leading
consultancy- Wellkang team based in both UK (England) & EU-27 (Ireland).
Wellkang team can help you under all Brexit scenarios!
Click here to get FREE Guide Now! |
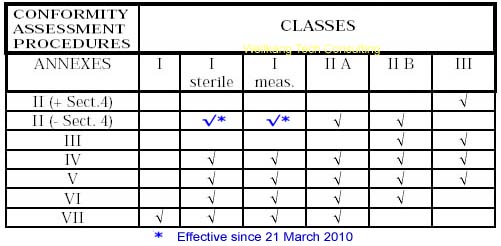
Steps for Class IIa medical devices compliance
- Classification: ensure the device is a Class IIa medical device.
- Choose Conformity Assessment Route: refer the flow chart below.
- Compile the Technical File.
- Obtain certification from a Notified Body
- Declaration of Conformity.
- Appoint an Authorised Representative. (Hold the Tech Files for inspection by the Competent Authority)
- Vigilance and Post Market Surveillance. (affix CE marking & market the products)
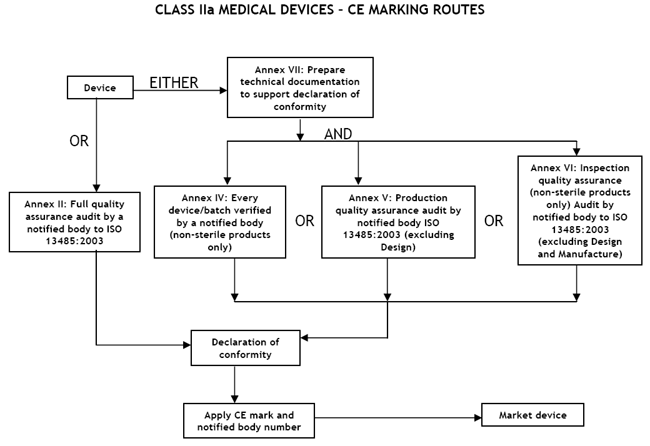
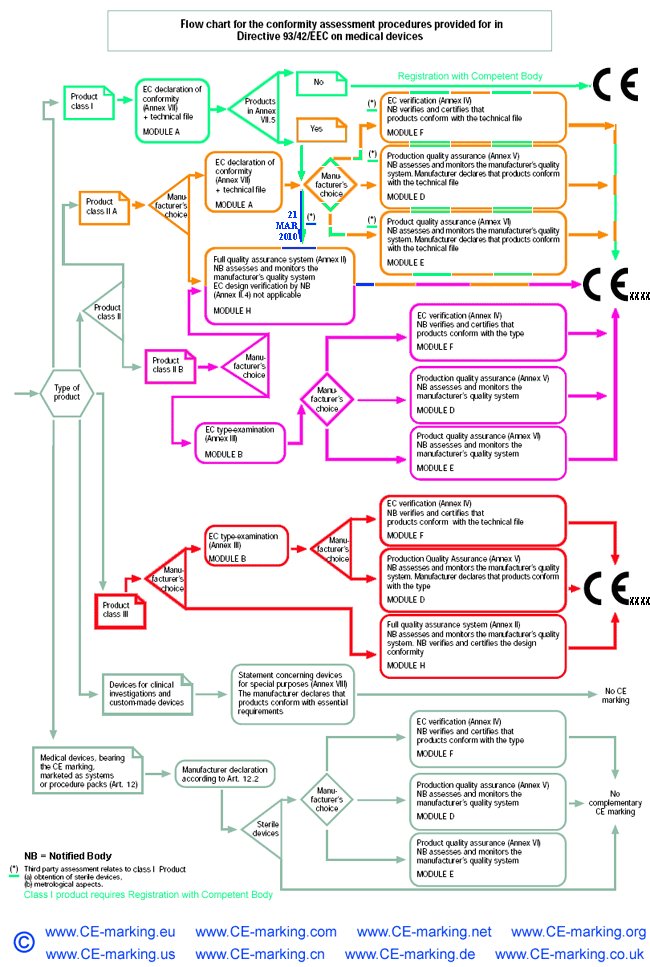
Class IIa Medical Devices: Conformity Assessment Routes
The conformity assessment routes for Class IIa Medical Devices
In the case of devices falling within Class IIa, other than devices
which are custom-made or intended for clinical investigations, the
manufacturer shall, in order to affix the CE marking,
- follow the procedure relating to the EC declaration of conformity
set out in Annex II (full quality assurance); in this case, point 4 of
Annex II is not applicable;
or
- follow the procedure relating to the EC declaration of conformity set out in
Annex VII, coupled with either:
- (a) the procedure relating to the EC verification set out in Annex IV;
or
- (b) the procedure relating to the EC declaration of conformity set out in
Annex V (production quality assurance);
or
- (c) the procedure relating to the EC declaration of conformity set out in
Annex VI (product quality assurance).
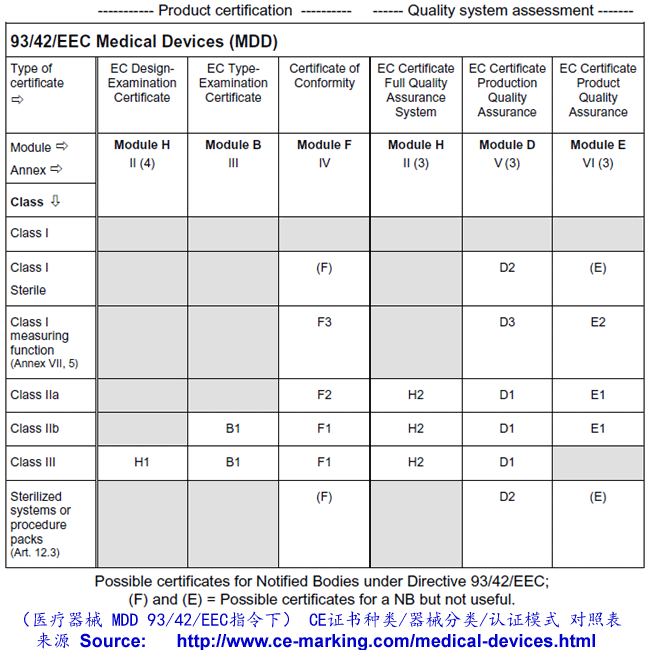
As for Class I, the manufacturer declares conformity with the provisions of the Directive
and Regulations and ensures that his products comply with relevant Essential
Requirements. However, for Class IIa products, this declaration must be backed up in all
cases with conformity assessment by Notified Body. This assessment may, at the
manufacturer’s choice, consist of:
- Examination and testing of each product or homogenous batch of products (Annex IV);
or
- Audit of the production quality assurance system (Annex V:) ISO 13485:2003 (excluding
Design) or
- Audit of final inspection and testing (Annex VI:) ISO 13485:2003 (excluding Design &
Manufacture) or
- Audit of the full quality assurance system (Annex II) ISO 13485:2003
Once the manufacturer has received certification from the Notified Body he may CE mark
his products and place them on the market.

FAQ/Q&A: Questions and Answers about CE Marking of Medical Devices
About CE Marking:
| Home | About Us | Contact Us | Copyright & Disclaimer | Privacy | Log-in | Order Now | |